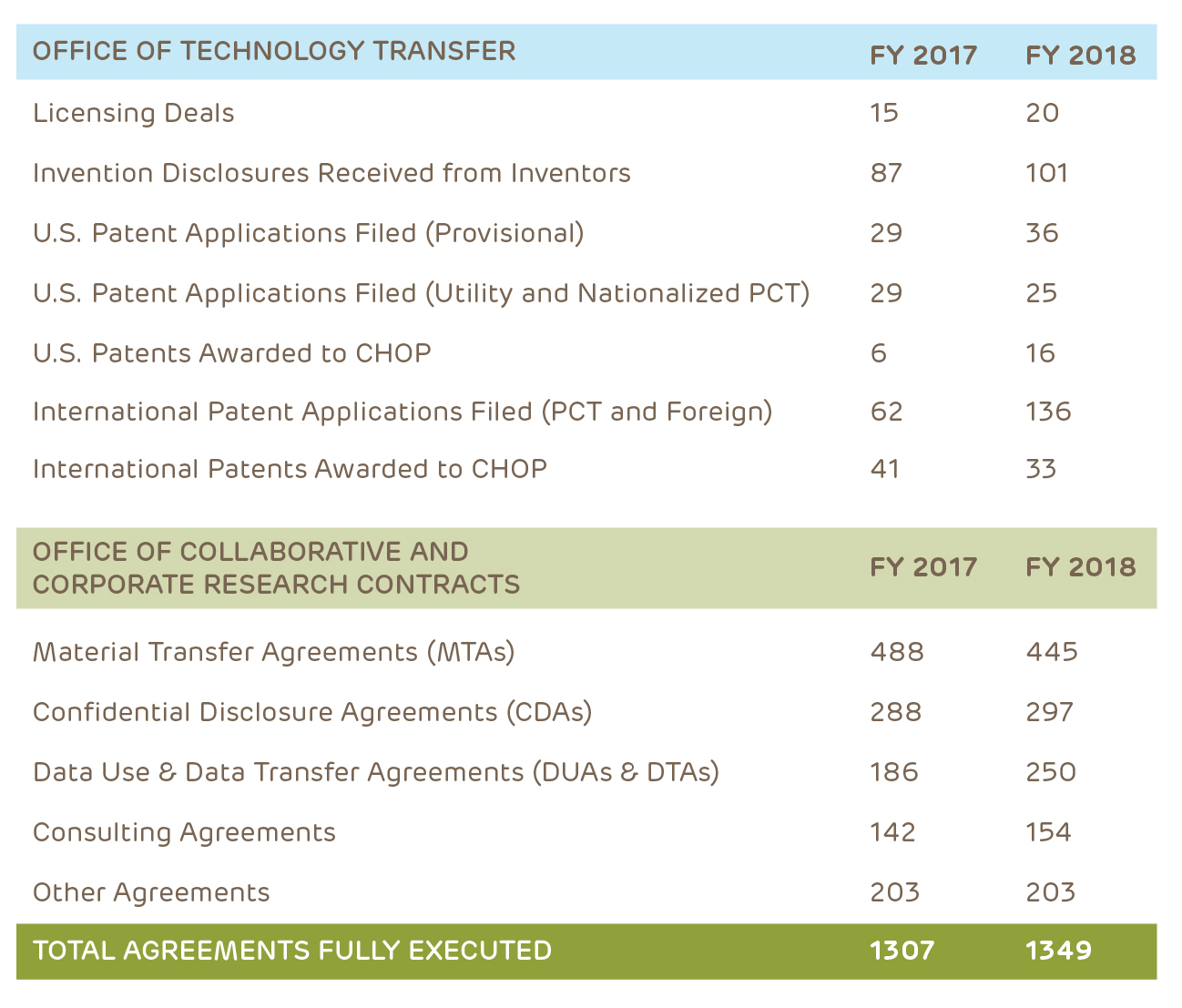
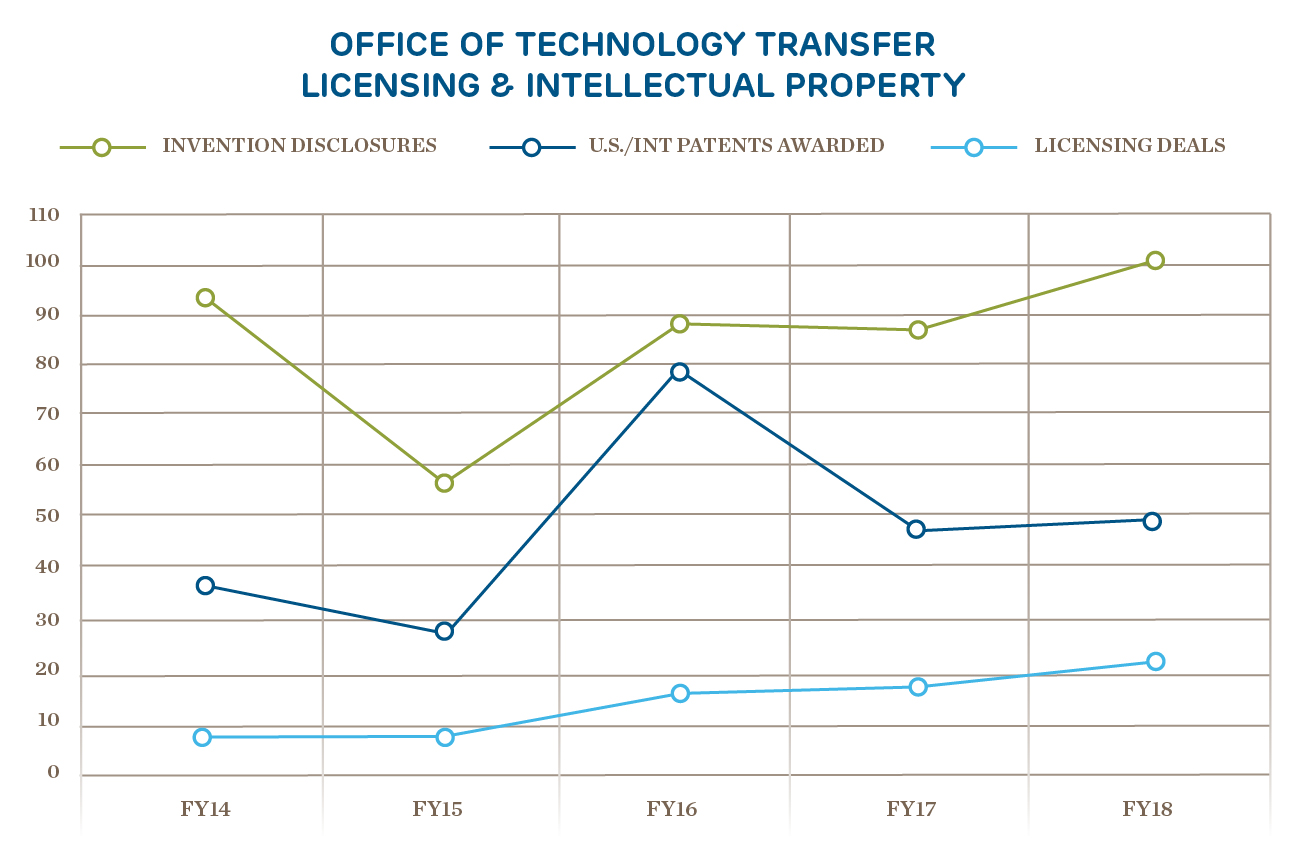
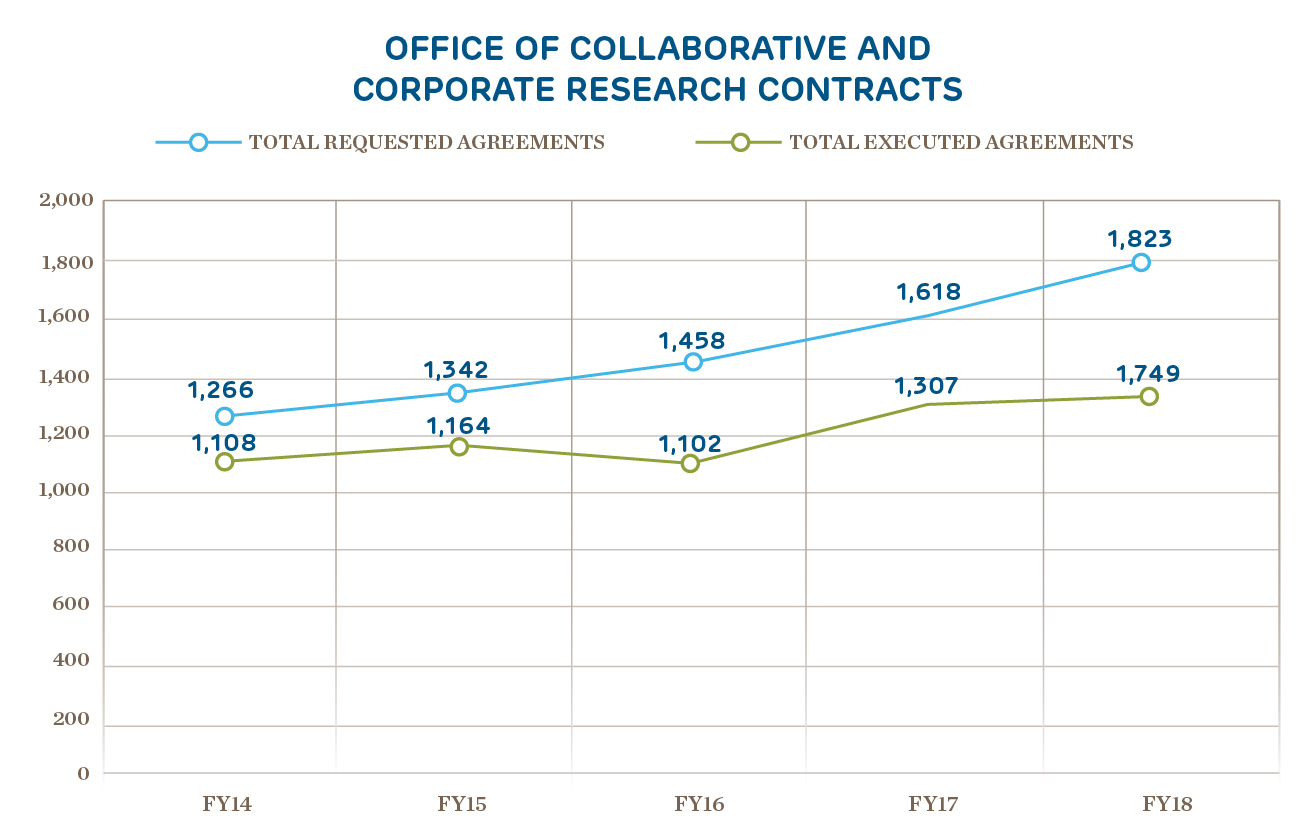
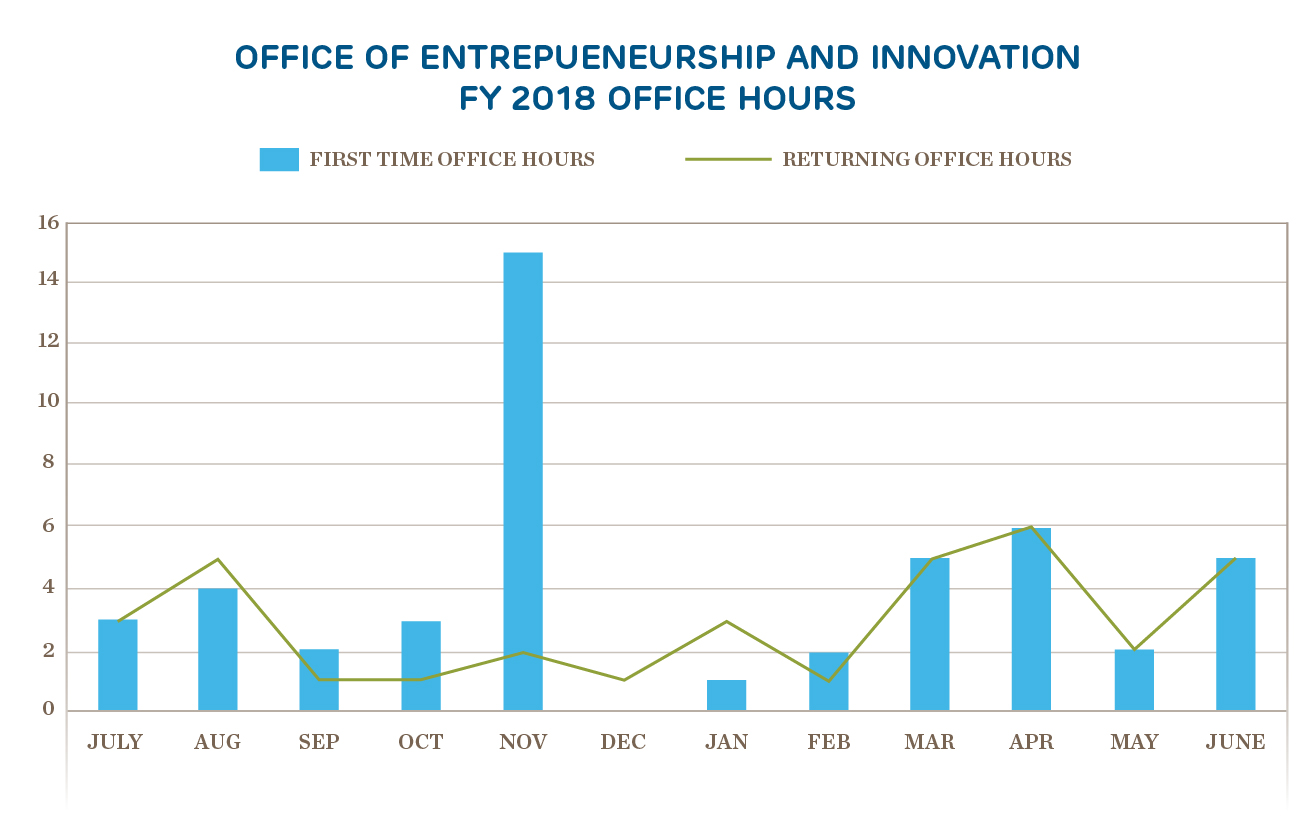
Innovation

CHOP to Address Key Challenges in Pediatric Clinical Trials
Nearly 60 percent of drugs used in children and 90 percent of those used in neonates are prescribed off-label, said Christopher Forrest, MD, PhD, a pediatrician at Children’s Hospital of Philadelphia. In addition, the lag from initial approval and labeling of a drug for adults and pediatric labeling stretches to nine years, a statistic that hasn’t changed over the last decade.
“A lack of an integrated, efficient pediatric clinical trials infrastructure, misalignment of stakeholder interests, and studies that are poorly designed and not feasible are all contributing barriers to trials for new drugs and devices for children,” Dr. Forrest said.
Change will be coming, though. CHOP serves as the coordinating center for PEDSnet, a national network that conducts observational pediatric research, said Dr. Forrest, PEDSnet principal investigator.
PEDSnet is allied with the Institute for Advanced Clinical Trials for Children (I-ACT for Children), an independent nonprofit organization that works to improve the planning and completion of pediatric clinical trials. In October 2017, the Food and Drug Administration awarded a $1 million cooperative agreement to I-ACT and its partners with the potential for $1 million annually for an additional four years to meet this goal.
“This is an excellent opportunity for PEDSnet because it allows us to expand from observational to prospective trials,” said Dr. Forrest of the funding.
Comprised of the nation’s leading children’s research hospitals, PEDSnet has established a novel real-world database of more than 6.5 million childrens’ electronic health record data that can be used for feasibility assessments, cohort discovery, natural history, and observational studies, he said.
“Our primary focus in trials is to establish real-world effectiveness of diverse healthcare interventions,” Dr. Forrest said. “PEDSnet will use these assets as it serves as the data and learning core for the Global Pediatric Clinical Trials Network, which aims to bring the kind of transformation necessary to engage diverse public and private stakeholders, re-engineer the current system, and catalyze existing expertise and resources.”

CHOP-Led Project to Ease Burden of Young Adults With Sickle Cell Disease
With a massive grant of nearly $8.5 million, researchers at Children’s Hospital of Philadelphia’s Comprehensive Sickle Cell Center, PolicyLab, and Northwell Health’s Cohen Children’s Medical Center will help smooth a traditionally difficult point in the lives of adolescents and young adults with sickle cell disease.
“This is an incredibly impactful grant, and I’m proud to be working on this,” said David Rubin, MD, MSCE, an attending physician and the founding co-director of PolicyLab at CHOP, who is one of two lead principal investigators of the project. “Youth with sickle cell disease have historically struggled in their transition to an adult world of medicine that’s often unprepared or unwilling to provide the care that they need.”
Kim Smith-Whitley, MD, clinical director of the Division of Hematology and director of the Comprehensive Sickle Cell Center at CHOP, also is a co-principal investigator. Young adults with sickle cell disease experience increased rates of death during the transition from pediatric to adult care, and they use more healthcare services than all other age groups, she said. Community health worker programs and mobile health applications could help this patient population, but it’s unknown to what extent these interventions actually improve their lives.
In “Community Health Workers and Mobile Health for Emerging Adults Transitioning Sickle Cell Disease Care” (COMETS Trial), funded by the Patient-Centered Outcomes Research Institute, researchers will compare the effectiveness of these two self-management support interventions vs. enhanced usual care to improve health-related quality of life and acute care use for transitioning youth with sickle cell disease, as well as identify and quantify mediators and moderators of intervention treatment effects. The COMETS Trial began recruiting patients in October 2018.
“The pressure to self-manage a chronic illness like this has never been greater,” Dr. Rubin said. “Being able to work among our partner sites to help achieve a successful transition for these youth — and build capacity for the youth who will come behind them — is a tremendous and humbling honor. If this work helps solidify the strategies not only in our backyard but serves as a template for other places in the country, all the better.”

Researchers Enlist Visual Eye-Tracking Algorithm to Enhance Concussion Diagnosis
Clinician-scientists at Children’s Hospital of Philadelphia lead pioneering research evaluating the impact of advanced eye-tracking tools to detect deficits in children and adolescents with concussions. By studying the utility of visio-vestibular exams (VVE) and automated eye-tracking assessments, our researchers work at the cutting-edge of developing objective and rapid tools for concussion diagnoses as well as for predicting recovery time. In 2018, these breakthroughs were recognized across the scientific community and the mainstream media.
“Many concussed youths have vision deficits, but if you aren’t looking for them, you won’t find them. If you don’t find them, you can’t treat them,” said Christina L Master, MD, FAAP, CAQSM, co-lead of the Minds Matter program and primary care sports medicine specialist at CHOP. “Having reliable practical clinical tools that can detect vision deficits soon after a concussion injury is critical to improving concussion care for kids.”
In a study published in the Clinical Journal of Sport Medicine, Dr. Master and colleagues found that 88 percent of 432 children in a concussion program presented with visio-vestibular deficits, such as problems with balance and tracking a moving object, that predicted prolonged concussion recovery. Furthermore, a team of CHOP researchers found that the VVE was a feasible tool for clinicians in high-volume, acute care settings and that the exam could help clinicians more accurately identify concussed patients.
Researchers in our Center for Injury Research and Prevention, in collaboration with other institutions, also found that an automated eye-tracking assessment proved to be a successful objective and noninvasive method for identifying concussion. As an alternative to more subjective and symptom-based assessments, tracking a patient’s binocular eye movements while watching a video are what Dr. Master calls “one tool” in a clinician’s diagnostic toolbox.
After a head injury, either of these innovative tests could establish an earlier diagnosis and thus help families, clinicians, and teachers develop the appropriate accommodations during recovery.
Learn more about the use of eye-tracking technology in concussion care.
Novel Approaches Create Healthier Futures for Patients Living With Inherited Blood Disorders
Patients living with sickle cell disease (SCD) and beta-thalassemia often face a journey of chronic symptoms and lifelong treatment just to go about their day-to-day activities.
Now, Children’s Hospital of Philadelphia researchers have helped identify a regulatory cell protein and genetic marker that hold promise for a dramatic impact in the lives of patients with these inherited blood disorders.
SCD study co-leaders Gerd A. Blobel, MD, PhD, a CHOP scientist and the Frank E. Weise III Endowed Chair in Pediatric Hematology, and Junwei Shi, PhD, assistant professor of cancer biology at the Perelman School of Medicine at the University of Pennsylvania, published their findings in the July 20 issue of Science. Their research details the discovery of an unexpected role of a signaling protein called heme-regulated EIF2α kinase (HRI) that regulates the transition of fetal-to-adult hemoglobin in red blood cells. When HRI function was selectively knocked out, the level of fetal hemoglobin in red blood cells increased — good news, as the SCD mutation only manifests after birth as fetal hemoglobin decreases in the body.
A critical finding was that the decreased sickling in red blood cells obtained from patients with SCD did not impair the viability or maturation of the cells, suggesting loss of HRI function could be well tolerated by patients. Moreover, these findings may have implications for the treatment of another inherited blood disorder involving abnormal hemoglobin, beta-thalassemia.
In a concurrent project, researchers at the CHOP Thalassemia Center may have uncovered an approach to drastically alter the lives of patients with the most severe form of the beta-globin mutation.
A paper published April 19 in The New England Journal of Medicine by Janet Kwiatkowski, MD, MSCE, assistant professor of pediatrics at CHOP, and co-authors shared the results of their work in gene therapy for the treatment of patients with transfusion-dependent beta-thalassemia.
In a follow-up study to further test the approach of previous researchers, two teams carried out companion Phase 2 clinical trials in which hematopoietic stem cells were collected from patients with severe beta-thalassemia. The normal beta-globin gene was added to the cells, which were then transferred back into the patients.
The results hold much promise for the future of treatment: Of 22 study subjects, nine with the most severe form of the disorder experienced a 74 percent reduction in their number of annual blood transfusions, and three patients in the most severe group were able to stop receiving transfusions entirely.
The team is currently recruiting for Phase 3 of the trial, which will further evaluate the efficacy and safety of autologous hematopoietic stem cell transplantation using LentiGlobin BB305.
These findings illustrate how CHOP researchers continuously investigate targeted, novel therapies to uncover the unexpected and deliver everyday breakthroughs for our patients.